Rainer HEINTZMANN
Image: Sven Döring, IPHT Jena.Prof. Dr. Rainer HEINTZMANN
Email: rainer.heintzmann@uni-jena.de
Phone: +49 3641-9-48350
As a professor of physical chemistry, Rainer Heintzmann is a member of the Faculty of Chemistry and Earth Sciences and the Faculty of Physics and Astronomy. He also heads a research unit at the Leibniz Institute of Photonic Technology in Jena. His main research direction has been high resolution optical microscopy and microscopy image processing.
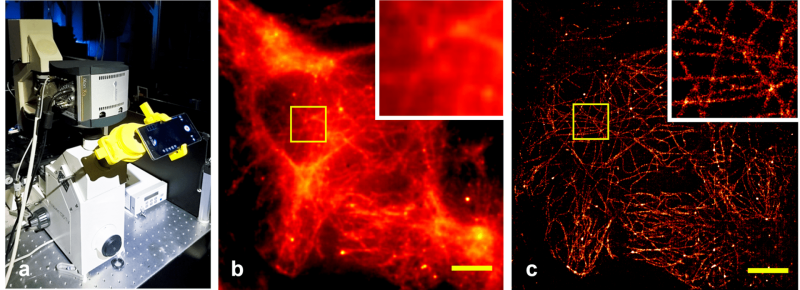
The group develops techniques to measure multidimensional information in small biological objects such as cells, cellular organelles, molecules or other structures of interest. We seek to unravel how molecules interact in living cells at specific places (e.g. inside organelles) and at well-defined times (e.g. after stimulation with other molecules). To reach this goal, we make use of molecules that can be switched between different fluorescent states by illumination at separate wavelengths. The transitions between the states can be driven into saturation and the arising non-linear dependencies can be used, e.g. by a technique called structured illumination, to reach a theoretically unlimited optical resolution.
Research Areas
The research of his group concentrates on the improvement of optical microscopy. Specific focus is currently placed on:
- Modern optical microscopy for biomedical research
- Research and implementation of super-resolved fluorescence microscopy through linear and non-linear structured illumination (SIM) and localization microscopy (dSTORM, DNA-PAINT)
- Hyper-spectral Raman-imaging for combined spatial and spectral information in medical research
- Affordable microscopes based on 3D-printig and smartphones for education and research
- Advanced image processing in microscopy
- Solving inverse problems in microscopy (e.g. image deconvolution and 3D-reconstruction)
Teaching Fields
Prof. Heintzmann teaches undergraduate courses in physical chemistry, as well as specialized courses on optics and image processing. These currently include:
- Light microscopy
- Biophotonics
- Image processing in microscopy
Research Methods
The methods developed in Prof. Heintzmann’s laboratories are applied, in collaboration with biologists, to biomedical problems. Available methods are:
- Fast structured illumination microscopy
- Localization microscopy (dSTORM, DNA-PAINT)
- Confocal microscopy
- Hyper-spectral Raman microscopy
- Affordable and flexible microscopes through 3D-printing and off-the-shelf electronics